Cement Composition
Cement
- A cementis a binder, a substance used in construction that sets and hardens and can bind other materials together.
- The most important types of cement are used as a component in the production of mortar in masonry, and of concrete, which is a combination of cement and an aggregate to form a strong building material.
Cement History
- Joseph Aspedin of Yorkshire (U.K.) was the first to introduce Portland cement in 1824 formed by heating a mixture of limestone and finely divided clay in a furnace to a temperature high enough to drive off the carbonic acid gas.
- In 1845, Issac C. Johnson invented the cement by increasing the temperature at which the mixture of limestone and clay were burned to form clinker. This cement was the prototype of the modern Portland cement.
- From then onwards, a gradual improvement in the properties and qualities of cement has been made possible by researchers in U.S.A., U.K., France and Germany
Cement Clinker
In the manufacture of Portland cement, clinker occurs as lumps or nodules, usually 3 millimetres (0.12 in) to 25 millimetres (0.98 in) in diameter, produced by sintering (fused together without melting to the point of liquefaction) limestone and alumino-silicate materials such as clay during the cement kiln stage.
Today cement finds extensive use in all types of construction works; in structures where high strength is required e.g. bridge piers, light houses, lofty towers, and large structures such as bridges, silos, chimneys. And also in structures exposed to the action of water, e.g. reservoirs, dams, dock yards etc. Cement mortar, concrete, reinforced brick work, artificial stones, plastering, pointing and partition walls are routinely used in buildings.
Portland Cement
- It is a cementing material resembling a natural stone quarried from Portland in U.K.

- Portland cement may be defined as a product obtained by finely pulverizing clinker produced by calcining to incipient fusion, an intimate and properly proportioned mixture of argillaceous and calcareous materials.
Care must be exercised in proportioning the raw materials so that the clinker of proper constitution may be obtained after burning.
Composition of Portland Cement
- The three constituents of hydraulic cements are lime, silica and alumina.
- In addition, most cements contain small proportions of iron oxide, magnesia, Sulphur trioxide and alkalis.
- There has been a change in the composition of Portland cement over the years, mainly reflected in the increase in lime content and in a slight decrease in silica content.
- An increase in lime content beyond a certain value makes it difficult to combine completely with other compounds.
- Consequently, free lime will exist in the clinker and will result in an unsound cement.
- An increase in silica content at the expense of alumina and ferric oxide makes the cement difficult to fuse and form clinker.
Chemical Composition of Portland Cement
The approximate limits of chemical composition in cement are given in following Table
Composition of Cement Clinker
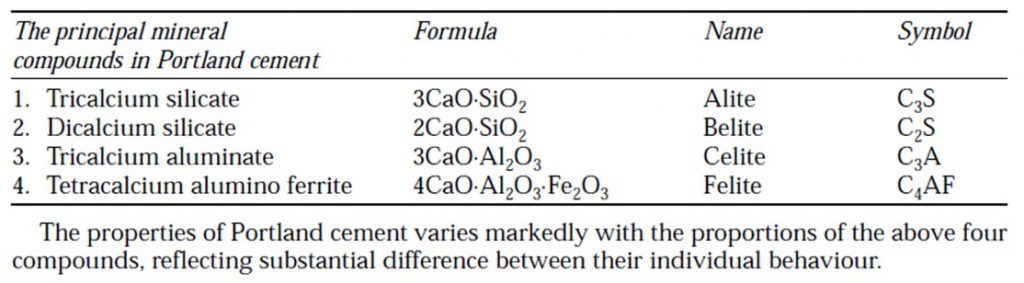
- The various constituents combine in burning and form cement clinker. The compounds formed in the burning process have the properties of setting and hardening in the presence of water.
- They are known as Bogue compounds after the name of Bogue who identified them. Le-Chatelier and Tornebohm have referred these compounds as Alite (C3S), Belite (C2S), Celite (C3A) and Felite (C4AF). The following Bogue compounds are formed during clinkering process.
Tricalcium Silicate
- is supposed to be the best cementing material and is well burnt cement.
- It is about 25-50% (normally about 40 per cent) of cement.
- It renders the clinker easier to grind, increases resistance to freezing and thawing, hydrates rapidly generating high heat and develops an early hardness and strength. However, raising of C3S content beyond the specified limits increases the heat of hydration and solubility of cement in water.
- The hydrolysis of C3S is mainly responsible for 7 day strength and hardness.
- The rate of hydrolysis of C3S and the character of gel developed are the main causes of the hardness and early strength of cement paste. The heat of hydration is 500 J/g.
Dicalcium Silicate
- is about 25-40% (normally about 32 per cent) of cement.
- It hydrates and hardens slowly and takes long time to add to the strength (after a year or more).
- It imparts resistance to chemical attack. Raising of C2S content renders clinker harder to grind, reduces early strength, decreases resistance to freezing and thawing at early ages and decreases heat of hydration.
- The hydrolysis of C2S proceeds slowly. At early ages, less than a month, C2S has little influence on strength and hardness. While after one year, its contribution to the strength and hardness is proportionately almost equal to C3S.
- The heat of hydration is 260 J/g.
Tricalcium Aluminate
- is about 5-11% (normally about 10.5 per cent) of cement.
- It rapidly reacts with water and is responsible for flash set of finely grounded clinker. The rapidity of action is regulated by the addition of 2-3% of gypsum at the time of grinding cement.
- Tricalcium aluminate is responsible for the initial set, high heat of hydration and has greater tendency to volume changes causing cracking.
- Raising the C3A content reduces the setting time, weakens resistance to sulphate attack and lowers the ultimate strength, heat of hydration and contraction during air hardening. The heat of hydration of 865 J/g.
Tetracalcilum Alumino Ferrite
- is about 8–14% (normally about 9 per cent) of cement.
- It is responsible for flash set but generates less heat.
- It has poorest cementing value.
- Raising the C4AF content reduces the strength slightly.
- The heat of hydration is 420 J/g.